
Retrieval of >15 oocytes has been shown to increase the risk of OHSS without improvement in the live birth rate.5
Clinical guidance recommends an individualized FSH starting dose based on factors predictive of successful outcomes.6
Rekovelle® is indicated for COS for the development of multiple follicles in women undergoing ART, such as an IVF or ICSI cycle. There is no clinical trial experience with Rekovelle® in the long GnRH agonist protocol.1
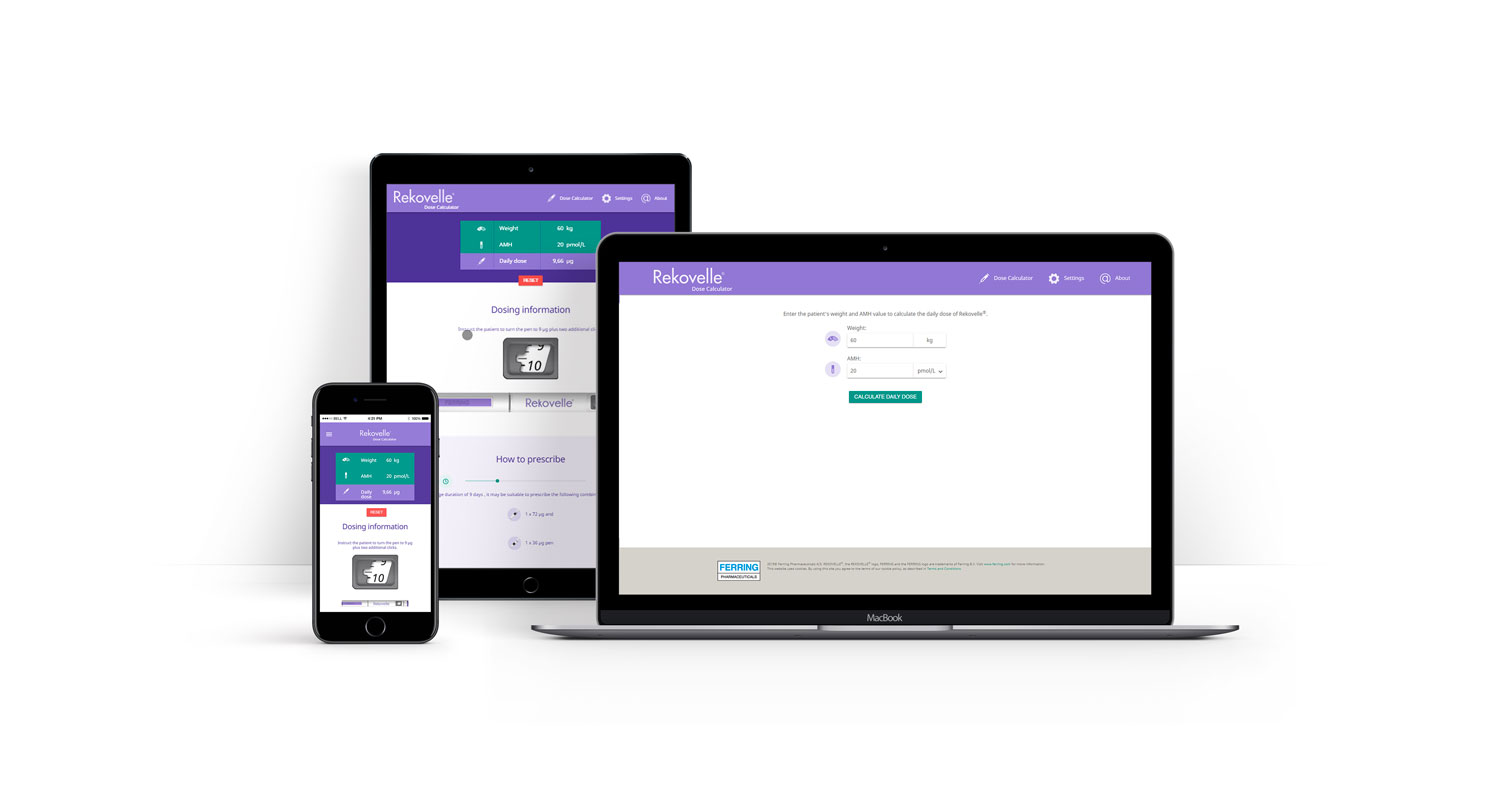
The only human recombinant FSH with an approved dosing algorithm designed for predictable ovarian response1,2,3
The validated Rekovelle® dosing algorithm uses AMH, as measured by the Roche Elecsys® AMH Plus assay, and body weight for dose calculations, with the aim of a targeted ovarian response and avoidance of extreme responses.*1–2
AMH is a robust predictor of ovarian response and the preferred biomarker for establishing an individualized dosing regimen.1,8–10
Rekovelle® is the first human recombinant FSH expressed in a human cell line.2,11
Rekovelle®’s unique glycosylation pattern leads to a distinct PK/PD profile.2,12
*The objective of the Rekovelle® dosing regimen was to obtain a predictable and adequate ovarian response associated with a favourable efficacy/safety profile. Adequate ovarian response was defined as 8–14 oocytes retrieved. In the ESTHER-1 study, it was predicted at trial enrolment that for the first treatment cycle, 42% of the individualized dosing group would yield an average of 8–14 oocytes, and this prediction was validated, with 43% attaining the predicted target yield. The stratified dosing regimen and the algorithm were prospectively validated in the registration trial (ESTHER-1) and approved by the European Medicines Agency (EMA).1,2
The validated Rekovelle® dosing algorithm uses AMH and body weight for dose calculations, with the aim of avoiding extreme responses.1,2


Rekovelle® delivers proven outcomes1
Unexpected extreme responses have both safety and efficacy implications.4,5
prospectively validated in a Phase 111 non-inferiority trial comparing the efficacy and safety of Rekovelle® and follitropin alfa* in first cycle patients undergoing ovarian stimulation for IVF/ICS1.t1
ESTHER-1 study design1
ESTHER-1 was a Phase III, randomised, controlled, assessor-blind, parallel-group, multicentre, multinational, non-inferiority trial comparing the efficacy and safety of Rekovelle® (n=665) and follitropin alfa (n=661) in first cycle patients aged 18–40 years undergoing ovarian stimulation for IVF/ICSI following a GnRH antagonist protocol. Study co-primary endpoints were ongoing pregnancy rate and ongoing implantation rate. Secondary endpoints included live birth rate, incidence of targeted and extreme ovarian response, and embryology. Safety and adverse events, including OHSS and/or preventative interventions for early OHSS, were also assessed. The non-inferiority limit for the risk difference between the two treatments was pre-specified at –8.0% for both co-primary endpoints as agreed with regulatory authorities.1
Rekovelle® delivers proven outcomes1
The co-primary endpoints of the ESTHER-1 study were met, demonstrating comparable efficacy to conventional stimulation with follitropin alfa at a starting dose of 150 IU fixed for the first 5 days, which could then be adjusted by 75 IU (to 450 IU maximum) based on individual response.1,2,
Ongoing pregnancy rate:*
Ongoing implantation rate:*
A favourable efficacy/safety profile1,2
With increasing levels of AMH, risk of OHSS and/or requirement for
preventative intervention differed in the two treatment groups.*2
ESTH ER-1 was neither designed nor powered to assess results based on secondary end points. Predefined secondary end points are used as a measurement to yield supportive evidence to evaluate additional effects relevant to informing the Rekovelle® individualised dosing regimen.
Targeted ovarian response with Rekovelle®*1
Differences were observed in the proportion of women achieving the
target response of 8-14 oocytes in the two treatment groups.*
Targeted ovarian response with Rekovelle®*1
Women with extreme responses*1
Women with extreme responses*1
OHSS-related outcomes in ESTHER-1*1
ESTHER-1 was neither designed nor powered to assess results based on secondary end points. Predefined secondary end points are used as a measurement to yield supportive evidence to evaluate additional effects relevant to informing the Rekovelle® individualised dosing regimen.
Summary of safety profile
The most frequently reported adverse reactions during treatment with Rekovelle® are headache, pelvic discomfort, pelvic pain, OHSS, nausea, adnexa uteri pain and fatigue. The frequency of these adverse reactions may decrease with repeated treatment cycles, as this has been observed in clinicat trials.2
Low risk of immunogenicity
Repeated treatment with Rekovelle® is not associated with decreased ovarian response and does not induce
immune-related adverse events.2
For a full overview of frequent adverse reactions in ail trials, please consult the Rekovelle® Summary of Product Characteristics.2


Optimise dosing for a predictable ovarian response*3
The Rekovelle® pre-filled pen is available in 3 different filled volumes:

With the validated Rekovelle® dosing
algorithm, calculate the appropriate dose
to obtain a targeted ovarian response*1

Optimise dosing from day 1 without
the need for adjustments1

The validated Rekovelle® dosing algorithm
helps predict total drug usage in a cycle,
thus reducing potential for wastet1
Rekovelle® offers personalised,
first cycle dosing based on:*1,2
- Body weight
- AMH (as measured with the Roche Elecsys® AMH Plus immunoassay companion diagnostic)t
Visit dose.rekovelle.com to get started
Delivers proven outcomes, with a favourable efficacy/safety profile1,2
Optimise dosing for a predictable ovarian response with Rekovelle® *1,2
Item Code: REK/463/2018/CH3a(1)b
Date of preparation: November 2018 FERRING, the Ferring Pharmaceuticals logo and other Ferring product names are trade marks of Ferring B.V. © Ferring B.V. 2018.
*The objective of the Rekovelle® dosing regimen was to obtain a predictable and adequate ovarian response associated with a favourable efficacy/safety profile. Adequate ovarian response was defined as 8–14 oocytes retrieved. In the ESTHER-1 study, it was predicted at trial enrolment that for the first treatment cycle, 42% of the individualised dosing group would yield an average of 8–14 oocytes, and this prediction was validated, with 43% attaining the predicted target yield. The stratified dosing regimen and the algorithm were prospectively validated in the registration trial (ESTHER-1) and approved by the European Medicines Agency (EMA).1,2 The validated Rekovelle® dosing algorithm uses AMH and body weight for dose calculations, with the aim of avoiding extreme responses.1,2 AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone 1. Refer to Ferring Pharmaceuticals REKOVELLE® Summary of Product Characteristics (Europe) 2017 for more detail; 2. Nyboe Andersen A, et al. Fertil Steril 2017;107:387–396; 3. Arce J-C, et al. In: Seifer DB, Tal R, editors. Anti-Müllerian hormone: biology, role in ovarian function and clinical significance. Hauppauge, NY: Nova Science Publishers 2016:83–102.
1 Arce JC et al. Nova Science Publishers, Inc. 2016,
2 Fauser et al. Human Reproduction Update 2008 , 14: 1-14
3 Iliodromiti et al. Hum Reprod Update. 2015 , 21: 698-710
4 Ref. Label: La Marca et al. Hum Reprod Update 2014, 20: 124–140
5 Nelson et al. Hum Reprod. 2007, 22: 2414-21
6 Nelson et al. Hum Reprod. 2009, 24: 867-875
7 Nyboe Andersen et al. Fertil Steril 2017, 107: 1
8 Olsson et al. J Clin Pharm. 2014, 54: 1299-1307
9 Rekovelle SUMMARY OF PRODUCT CHARACTERISTICS, 1: 1-114 .
10 Steward et al. Fertil Steril 2014, 101: 967–973
11 World Intellectual Property Organization 2009














